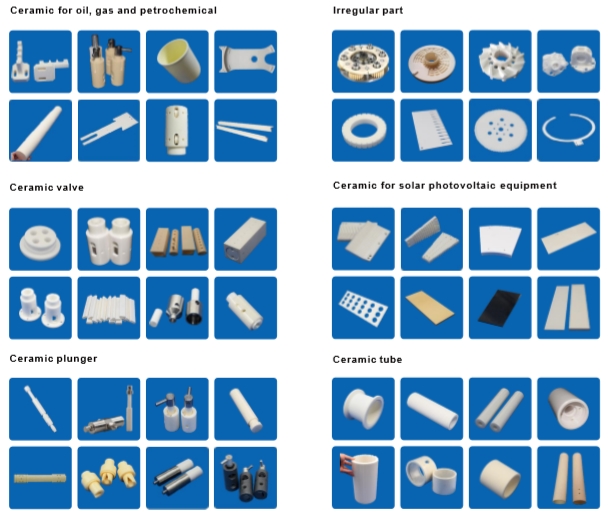
Alumina Ceramic Structural Parts
Physical properties of alumina (Al2O3):
White solid, high melting point (2054℃), boiling point 2980℃, often used as refractory material; it is amphoteric oxide. The main component of the gems we commonly see is alumina.
The reason for the various colors is the clinical manifestations and signs of metal oxides in gems. For example, ruby is red because it contains a small amount of chromium, and sapphire is blue because it contains a small amount of iron and titanium.
The main component of ore corundum in industrial production is α-alumina, whose hardness is second only to diamond and has a wide range of uses. Amphoteric oxides: oxides that can react with strong acids and strong bases to form salts and water. Al2O3+6HCl==2AlCl3+3H2O, Al2O3+2NaOH==2NaAlO2+H2O. Al2O3 is the raw material for industrial smelting of aluminum. Due to the high melting point of alumina, it is difficult to melt during electrolysis. Therefore, the smelting of aluminum until 1886, the American scientist Hall It is found that adding cryolite (Na3AlF6) to alumina reduces the melting point of alumina to about 1000 degrees, and the rapid development of aluminum smelting, and aluminum and its alloys are widely used.
Physical properties of alumina ceramics:
Among oxides with a melting point of more than 2000°C, alumina ceramics is the most flexible and cheap material. Alumina ceramics is a ceramic material with alumina (alumina) as the main body. Alumina ceramics has high mechanical strength, high hardness, low high-frequency dielectric loss, and because of its wide source of raw materials, relatively cheap price, and mature processing technology, it is widely used in electronics, electrical appliances, machinery, textiles and Aerospace and other fields. This also established its high position in the field of ceramic materials. It is reported that alumina ceramics is currently the most used oxide ceramic material in the world.

 Moble: +86 18122974730
Moble: +86 18122974730 Phone: +86 0769-85090316-8038
Phone: +86 0769-85090316-8038 Email: admin@cerampart.com
Email: admin@cerampart.com Skype: admin@cerampart.com
Skype: admin@cerampart.com Wechat: +86 18122974730
Wechat: +86 18122974730